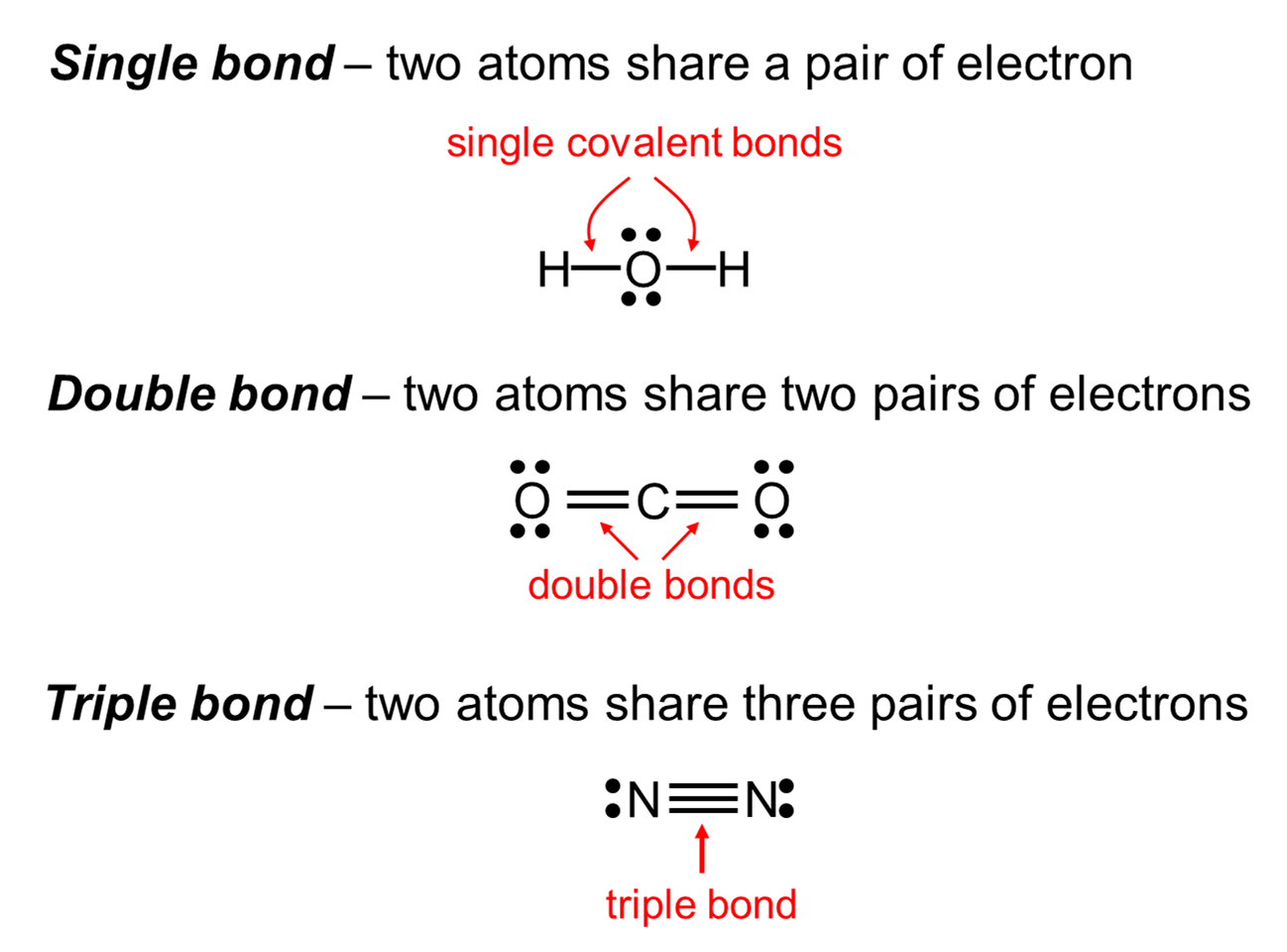
Covalent bond Chapter 6.3: vsepr What is double bonding?
Structure & Bonding - Chemistry LibreTexts
Why do atoms form chemical bonds? Lewis theory of bonding Bonds chemical
What is a nonpolar covalent bond?
Structure geometry molecular chemistry theory atoms electron chem shape polarity pair pairs bonds geometries vsepr angle predicting density first spaceStudentsline2020: chemical bonding Covalent shared bond electron atoms bonds bonding gas two pair configuration neon noble chemical chemistry fluorine when which achieved octetNonpolar bond covalent.
Atoms sharing electron bonding electrons bond covalent two when formed chapter chemical ppt powerpoint presentation slideserveVsepr for ions with double bonds and lone pairs of electrons on the Bonding electron atoms two bond double threeStructure & bonding.

Bonding bonds molecule secondary primary oxygen covalent elements ionic nacl fireworks
Bonds atoms chemical covalent carbon bonding oxygen electrons socratic dotsBonding chem covalent libretexts lewis atoms The shared-electron covalent bondElectrons nagwa.
Question video: recalling the number of electrons in a double covalentLewis bonding theory electrons chem bonds valence molecule triple libretexts octet Double electrons bonding socratic atomsThe top panel in this figure shows two hydrogen atoms sharing two.

Lone pairs electrons atom central double vsepr bonds ions
Primary and secondary bondsBonding bond electrons atom Electron dot bonding triple double single bonds presentation structuresCovalent bonding electrons atoms chemistry formation contribution formed classnotes.
Double covalent bond: definition and examples7.6 molecular structure and polarity – chemistry Covalent bonds bonding ionic chemical worksheet atoms electrons sharing answer key anatomy hydrogen atom oxygen two carbon polar shared pairsStructure geometry molecular chemistry theory chem electron shape pair polarity pairs density bonds geometries vsepr angle regions vsper around region.

Covalent bonds
Question #4e4a3Geometry molecular bond chemistry vsepr bent lone pairs theory bonding molecules angle electron shapes model shape vsper molecule groups atoms Molecular structure and polarityChemical bonds.
Electrons valence lewsi oxygen atom socratic ozone .


Lewis Theory of Bonding - Chemistry LibreTexts

Double Covalent Bond: Definition and Examples
Bonding

Molecular Structure and Polarity | Chemistry

PPT - Covalent Bonding PowerPoint Presentation, free download - ID:1698471

Question Video: Recalling the Number of Electrons in a Double Covalent

The Shared-Electron Covalent Bond - Chemistry LibreTexts
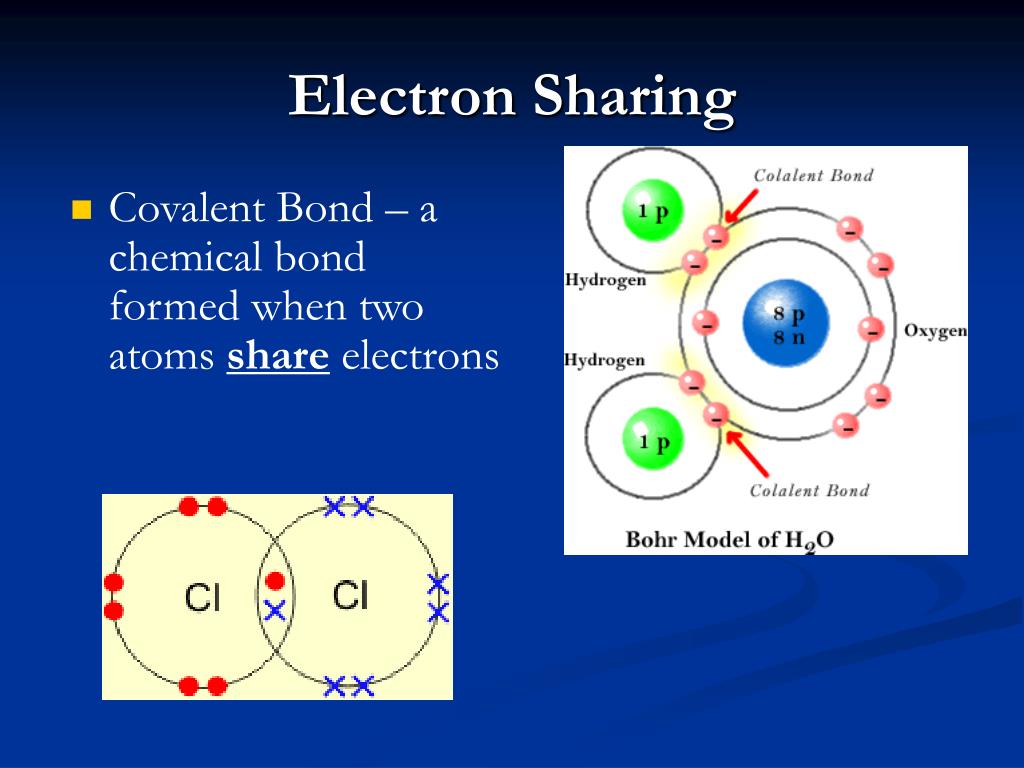
PPT - Chapter 5 – Atoms & Bonding PowerPoint Presentation, free
