What is the electron configuration of chlorine? Orbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcom 5.14: aufbau principle
12.1.4 State the maximum number of orbitals in a given energy level
Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer Difference between 4f and 5f orbitals Electronic orbitals
12.1.4 state the maximum number of orbitals in a given energy level
Orbitals sublevels electrons3.7: electron arrangement- the quantum model Orbitals electron orbital orbitali electrons atomici atomic atoms quantici numeri biopills shapes atom arrangement chem libretexts directional toppr atomo foundHow many orbitals are in the n = 3 level?.
2.2: electron configurations8.3 development of quantum theory – chem 1114 – introduction to chemistry Electron configurationsSpdf orbitals : parsing spdf orbital hybridization and simple bonding.

Aufbau principle electron orbital atomic filling energy arrangement atoms ck electrons chem 1s 2s 2p foundation 3s libretexts bromine atom
Orbitals sublevel shapes sublevels 2s 3s axis identical madeOrbitals chemistry electron atoms subshell order table atomic configurations periodic number structure quantum subshells electronic electrons energies which full configuration Maximum number orbitals energy level given stateOrbital orbitals atomic 5f quantum number electron magnetic 4f shapes chemistry types difference between seven atom shape different chemie their.
Orbitals electron electronic single orbital atomic nodes shapes electrons angular quantum atom diagram chemistry orbitales chemwiki radial atoms diagrams thereOrbitals shapes atomic quantum chemistry atoms chem wave electrons theory shape electron atom model numbers chart figure shaped space orbital 4f orbitals 5f difference between1.5-sublevels orbitals and electrons.
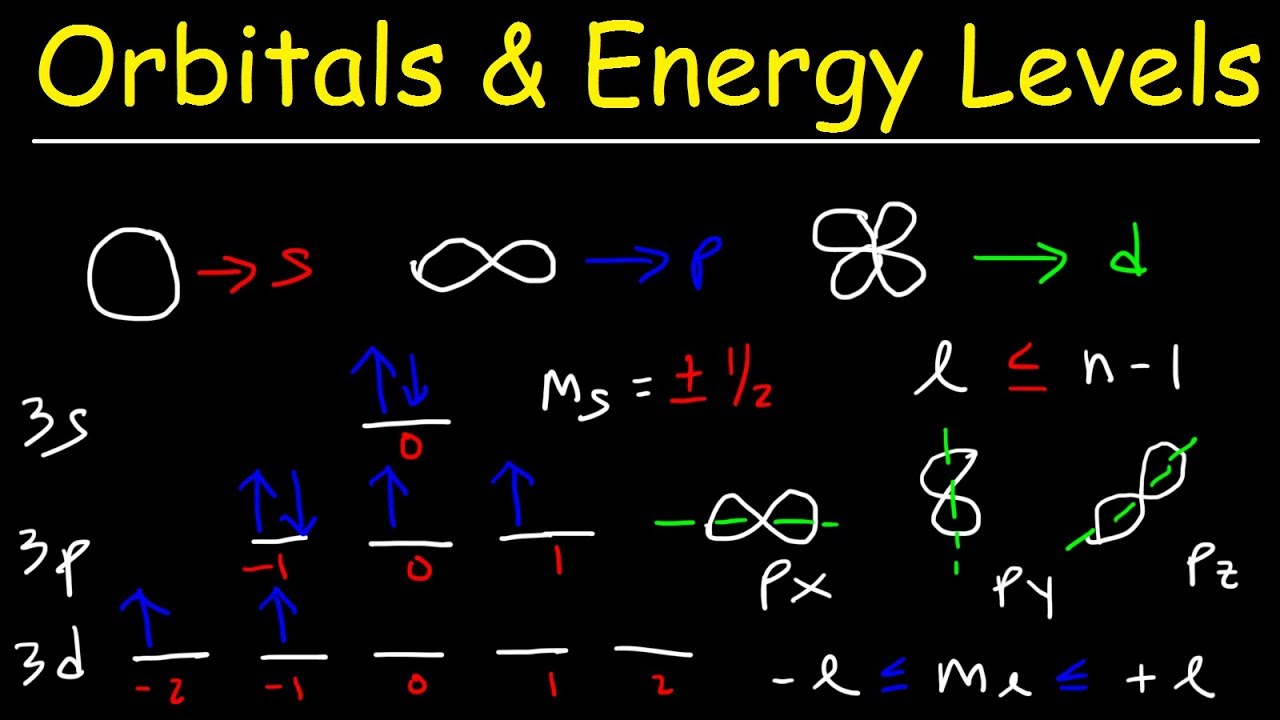
Shapes of orbitals and their types
Electron configurations orbitals sublevel each has line orbital chemistry box within own its theseElectrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does Shapes of orbitals and sublevels.
.


Electronic Orbitals - Chemwiki

Difference Between 4f and 5f Orbitals | Definition, Seven Orbitals and

3.7: Electron Arrangement- The Quantum Model - Chemistry LibreTexts

8.3 Development of Quantum Theory – CHEM 1114 – Introduction to Chemistry

What is the electron configuration of chlorine? | Socratic

Shapes of Orbitals and their Types | Chemistry Skills

how many orbitals are in the n = 3 level?
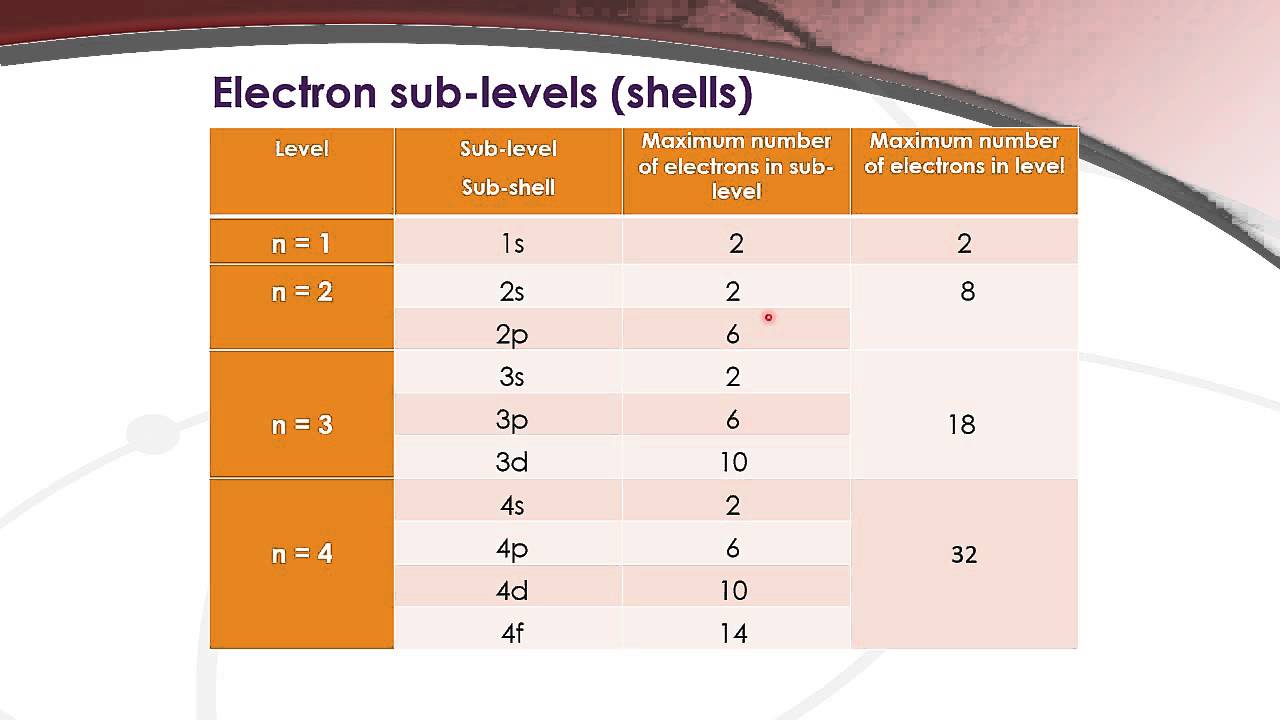
12.1.4 State the maximum number of orbitals in a given energy level

Electron Configurations | CK-12 Foundation
