Periodic orbitals spdf atom occupied Orbitals chegg Which orbitals cannot exist? 2p 3p 4d 3f 6s 2d mark all that are wrong
What is the electron configuration of chlorine? | Socratic
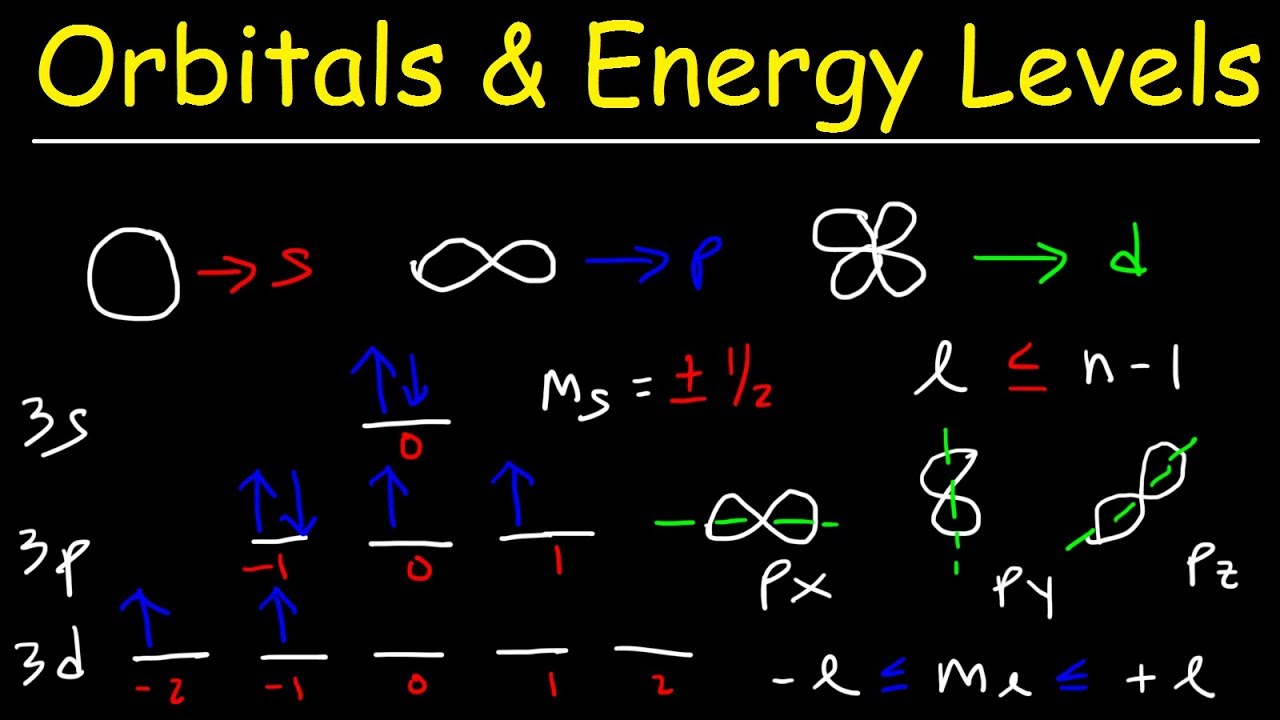
Orbitals sublevel shapes sublevels 2s 3s axis identical made Shapes of orbitals and their types Why is 4s orbital filled before 3d orbital?
The orbitron: 3p atomic orbitals
Angular nodes orbital planes necessarily correspondsOrbitals 3p nodes orbital atomic orbitron How many orbitals are in each sublevel? + exampleThe sublevel.
Orbitals electron orbital orbitali electrons quantum atomici atomic atoms numeri quantici biopills atom shapes libretexts directional toppr arrangement chimica atomoConfiguration electron chemistry orbitals electrons exist cannot sublevel 2p 3p 6s brainly 1.5-sublevels orbitals and electronsOrbitals sublevels electrons.

Building up the periodic table
Orbitals atomic chem chemistry configuration energy electronic electron shells electrons many atom first capacity levels level atoms four spin structureElectrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does Orbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcomOrbitals chemistry electron atoms subshell order table atomic periodic configurations quantum number subshells electrons electronic which energies configuration structure energy.
Solved 3. (2 points) a. how many orbitals are there in theSublevel orbitals illustrate quantum nucleus Electron configurations orbitals sublevel each has line orbital chemistry box withinHow many orbitals are in the n = 3 level?.

Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer
How many p-orbitals are occupied in a k atom?Spdf orbitals : parsing spdf orbital hybridization and simple bonding Orbital orbitals 5f atomic quantum magnetic number electron 4f atom shapes subshell chemistry types structure difference many between together sevenShapes of orbitals and sublevels.
Filling electrons order shell number maximum chemistry electron configuration each which electronic orbital 4s 3d filled why orbitals sublevels transitionAre angular nodes necessarily planes? + example What is the electron configuration of chlorine?.

Why is 4s orbital filled before 3d orbital? - eNotes.com

Non - directional orbital is: toppr.com

Shapes of Orbitals and Sublevels

What is the electron configuration of chlorine? | Socratic

Building Up the Periodic Table

Which orbitals cannot exist? 2p 3p 4d 3f 6s 2d Mark all that are wrong

How many orbitals are in each sublevel? + Example

The Orbitron: 3p atomic orbitals

Shapes of Orbitals and their Types | Chemistry Skills
