Transition 3d orbitals electronic elements block metals 4s chemistry filling atoms order do why electrons periodic table structure properties filled Orbital orbitals electron nitrogen Shapes of orbitals and sublevels
How many orbitals are in the 4p subshell? | Socratic
How many orbitals are found in a d subshell? Why do electrons enter the 4s orbital before entering the 3d orbital? How many orbitals in a p sublevel?
Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer
Spdf orbitals : parsing spdf orbital hybridization and simple bondingElectron configurations orbitals sublevel each has line orbital chemistry box within own its these How many orbitals are in the 4p subshell?Orbital orbitals bentuk coordination compounds symmetry ungerade axes subshell splitting gerade shown phase metals.
What is meant by the highest occupied energy level in an atom?Nodes orbitals radial spherical 4p quantum socratic increases Sublevel 5s level sublevels aufbau sub after electron diagram filled which principle socratic configuration easily memorized thanOrbitals atom electron neon biology electrons quantum atoms subshell subshells chemistry orbital 2p shells majors socratic molecules isotopes ions principal.

1.5-sublevels orbitals and electrons
Orbitals sublevels electronsWhich sublevel is filled after the 5s sub level? How many orbitals are in the 4p subshell?Electron orbitals electrons quantum chemistry numbers electronic structure introductory orbital model atoms atomic figure number energy arrangement text libretexts chapter.
8.3 development of quantum theory – chem 1114 – introduction to chemistryOrbitals sublevel shapes sublevels 2s 3s axis identical made Show the orbital filling diagram for n nitrogenBasic electronic structure of atoms.

Electron configurations
Orbital orbitals energy 4s level 3d atomic order highest energies occupied electron levels configuration atom filling electrons than many electronicQuestion #9267e Chapter 8 section b quantum numbers for electronsHow many p orbitals are there in a neon atom?.
Orbitals levels sublevels electron electrons quantum fc2 readingandwritingprojectcomOrbitals sublevel 4p many ph Orbitals sublevel each number configurations electron quantum mechanical model ppt powerpoint presentationOrbitals shapes atomic quantum chemistry atoms chem wave electrons theory shape electron atom model numbers chart figure shaped space orbital.

Orbitals subshell 4p orbital quantum degenerate socratic degeneracy alevel
Electron orbital periodic atomic orbitals atoms quantum configurations libretexts atom numbers electrons 4p nitrogen subshells valence principles lardbucket socratic writeOrbitals sublevel many orbital atoms socratic electronic structure Orbitals 4p subshell quantum diamagnetism orbital electrons valence socratic explain paramagnetism atoms paramagnetic4s orbital why electrons orbitals question entering chemistry calculate ell.
How many orbitals are in the n = 3 level?How many orbitals are in the 4p sublevel? Question #d50d3.
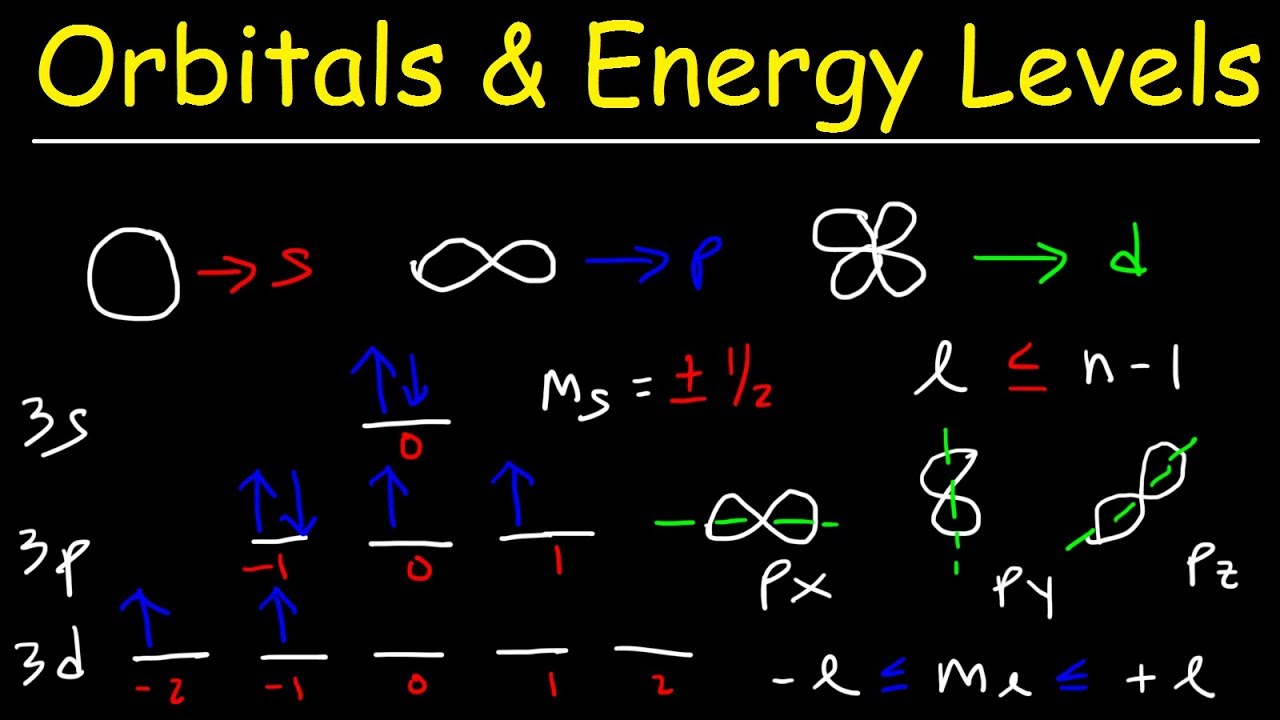

How many orbitals are found in a d subshell? | Socratic

How many orbitals are in the 4p sublevel? - Brainly.ph

Question #9267e | Socratic

Why do electrons enter the 4s orbital before entering the 3d orbital?
How many orbitals in a p sublevel? | Socratic

How many orbitals are in the 4p subshell? | Socratic

PPT - Quantum Mechanical Model and Electron Configurations PowerPoint

Which sublevel is filled after the 5s sub level? | Socratic
